Next-level imaging born and developed at Brown lets us see bodies in motion.
Behind an unmarked beige door in Brown’s Biomedical Center, you’ll find an unlikely menagerie. On any given day, you could encounter turtles, snakes, pigs, ducks, goats, sheep, or guinea fowl, each surrounded by a staggering array of medical machinery.
“You never know what you’ll see in here. One time we had an alligator running on a treadmill. Today, we’re looking at athletes’ knees,” says Braden Fleming, PhD, the Lucy Lippitt Professor of Orthopedics and a professor of engineering.
Those animals—and humans—are all here because of that machinery’s unique ability: it can peer inside moving tissue, revealing how bone, muscle, ligaments, and cartilage work together to let living organisms walk, breathe, and eat.
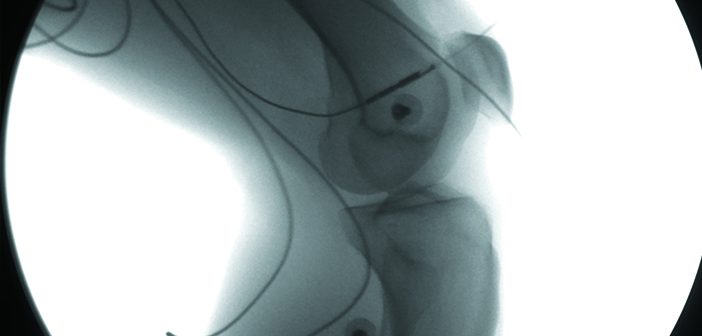
The room, called the W. M. Keck XROMM Facility (for “X-ray Reconstruction of Moving Morphology”), is one of the first of its kind. It certainly looks unique: flanking a thick blue rubber mat on the floor, two huge fluoroscopes—a sort of x-ray movie camera—are mounted on beefy metal structures. A cross from them, suspended from the ceiling on thick steel arms, hang powerful x-ray sources, served by a special 440-volt electrical system. The two fluoroscopes record moving x-ray images from different angles, giving researchers two distinct views of a subject’s bones. By combining data from the images, the scientists can effectively map a joint’s motion in three dimensional space.
Surprisingly, XROMM only requires minor modifications to existing x-ray machinery, says Elizabeth Brainerd, PhD, a professor of biology and of medical science, who founded the center in 2006. Instead of the standard 30 frames per second that most fluoroscopes capture, the XROMM system is set to record hundreds, even thousands of frames each second, letting scientists see minute changes within a joint as it flexes and twists.
For Brainerd, XROMM offers a means of conducting research that she had previously only dreamed of. “There have been a number of problems in my career that I’ve been interested in, but I sort of needed to give up on because there just wasn’t the right technology available to be able to study them,” she says. “Lung biomechanics, like how ribs move against each other as animals breathe—that’s really been puzzling for centuries. How do humans and animals use muscles to expand the rib cage using muscles that only pull instead of push? We just haven’t had the ability to measure rib shape and rib motion together in 3-D.”
That’s where the real power of XROMM comes in, she says. It not only lets researchers understand how individual points on each bone move; it also lets them reconstruct a fully accurate computer model of a joint with sub-millimeter accuracy. Using a CT scanner, scientists can create a static 3-D image of a subject’s bones, then call on powerful animation software to superimpose it onto video x-rays taken in the XROMM facility.
“It’s like a shadow puppet. You use the x-ray video to control the motion of the bones generated from the CT scan model,” Fleming says.
The result looks like something out of Hollywood—a ghostly pig skull or disembodied iguana spine, floating
against a white background on a computer screen, its component parts moving precisely the way they do in a living creature.
The technique opens up vast possibilities for researchers, Brainerd says. It can be adapted to capture images of fish feeding in water tanks; animals burrowing in sand; even birds in flight within small wind tunnels, providing images and data that would otherwise be nearly impossible to obtain.
The real kicker, though, is that the system may not just shed light on living creatures. It may also help reconstruct the evolution of animal motion, going all the way back to the early dinosaurs.
Stop Motion
Stephen Gatesy, PhD, should know. He’s an evolutionary biologist, and co-developed XROMM with Brainerd. He says that understanding the way bones functioned in extinct creatures has been an ongoing and somewhat quixotic quest.
“There’s a long tradition in paleontology and anthropology where you grab two bones and rub them together and say, ‘I guess they worked like this,’” says Gatesy, a professor of biology and of medical science. “But for anything more complex than a simple ball and socket joint, there’s a lot we don’t understand.”
To get around this limitation, Gatesy and other evolutionary biologists often look to modern living animals to find bone structures similar to extinct species. Alligators, for example, have hip joints that are tantalizingly close to dinosaur species like sauropods, huge land-dwellers that walked on four limbs (think Brontosaurus). Likewise, the joints of modern birds may reveal some details about how animals like T. rex once moved.
Measuring the skeletons of birds while in motion hasn’t always been easy, though. As a graduate student in the 1980s, Gatesy observed his mentors, including T ed Goslow, PhD, professor emeritus of biology, take some of the first xray videos of starlings in flight, offering a then-unprecedented look at what each bone and joint was doing along the way. Yet even that breakthrough provided only limited information—the two-dimensional x-ray effectively flattened each joint, superimposing every bone over its neighbors, creating images that were extremely hard to parse.
To squeeze more data from those x-rays, Gatesy realized, he would have to reach for techniques he’d picked up in his childhood hobby: creating stop animations with a super-8 film camera. “Rather than picking out points from a [2-D] x-ray—there’s the shoulder, wrist, elbow—I thought, if we have 3-D models of bones, we should be able to rig that up into an animatable puppet, and re-align the 3-D model to a 2-D x-ray view.”
Initially, Gatesy’s colleagues did that with dried starling skeletons, carefully posing each bone to match its alignment in a single frame of the x-ray video, then measuring its angle with a protractor and calipers. Later, he adapted professional animation software to do the job, painstakingly drawing a digital “stick figure” of each bone as it appeared. Working frame by frame in this way, he could eventually create a basic 3-D computer animation of a limb or joint.
It was slow work, but Gatesy plugged away at it in his lab for years. Once Beth Brainerd arrived at Brown in 2006, however, she began to grasp the potential of his work. Together they received seed funding from Brown and then a grant from the National Science Foundation to develop new hardware and software, and XROMM was born.
“Things that used to take us a whole morning suddenly took us 30 seconds,” says Gatesy. “It was Beth that really promoted looking outward to other colleagues and institutions to share our knowledge. Without that, I would still be doing it myself, hoping I got it right.”
Brainerd and Gatesy combined their 3-D software efforts with a pair of x-ray fluoroscopes taken from a hospital floor. Thanks to funding from the Keck Foundation, their original prototype has since grown to its current roomsized device, complete with a wall of high-powered computers. Through each iteration, Brainerd says, the equipment has become more flexible and versatile, yet still remained safe enough to use on human subjects.
The Whole Picture
That’s good news for J. J. Trey Crisco, PhD, the Henry F. Lippitt Professor of Orthopedics and a professor of engineering. Crisco has helped study prosthetic devices for partial amputees, who often encounter ill-fitting artificial limbs. Normally, those prostheses are bolted to a hard plastic or resin socket that slides onto a patient’s residual limb—but in many cases, the socket doesn’t move precisely with the remaining tissue, resulting in poor control, skin breakdown, and other serious problems for its wearer.
“When you move the prosthesis, you’re really controlling it by moving residual bone inside a limb,” says Crisco, who directs the Department of Orthopaedics’ Bioengineering Laboratory. “Even though there’s muscle between the bone and a prostheses’ socket, it’s the bone that’s driving motion. You want to minimize times when the bone is moving, but the socket isn’t.”
With XROMM, that may be possible in a matter of minutes. Crisco has been able to use the system to see exactly where a patient’s bone sits inside a prosthetic socket, offering minute detail on how well it fits their residual limb. Using that data, he says, prosthetics companies will be able to both improve future designs, and better customize existing ones to patients’ needs.
Crisco is also using the XROMM system to look at internal prosthetics, like those used to replace diseased or damaged joints. Although artificial shoulder, hip, and knee joints all work very well, he says, implants like prosthetic wrists are decidedly lacking. Compared to most joints in the human body, the wrist is incredibly complex, with eight separate bones and dozens of interlocking tendons and ligaments—but existing artificial versions are usually made up of just a single articulation. In many cases, that mechanism cannot approximate the motion of the entire wrist joint, causing unnatural motion that can lead to severe arthritis.
“There is very little understanding of how all those bones work together, how the forearm works together with the wrist,” Crisco says. “We know certain wrist bones and ligamental structures are critical to wrist function, but as for what each bone does? We don’t have the full picture by any means.”
By bringing patients into the XROMM facility, Crisco adds, it will be possible to measure all eight wrist bones—and their supporting tendons and ligaments—as they move their wrist and forearm through tasks of everyday living.
“XROMM will be critical to advancing our understanding of the wrist,” he says. “We think it could lead to new
therapies after injuries, and better joint replacement designs in the future.”
Popular Mechanics
But that future is a long way off. For now, researchers still struggle to make sense of why even simple joints like the knee often deteriorate after an injury.
Jillian Beveridge, PhD, a postdoctoral research fellow in the Bioengineering Laboratory, has a personal stake in that question. A s a varsity athlete, she tore the anterior cruciate ligament (ACL) in both of her knees, requiring painful surgery and rehabilitation. Although in her case the damage was successfully reconstructed and she has been able to remain physically active, she says it made her wonder why people with identical injuries—and identical treatments—can have vastly different outcomes. While some patients recover as if the injury never happened, others experience crippling pain and osteoarthritis years later.
As a graduate student, Beveridge began to pick apart that question by recreating the injury in animal limbs. “We found that damage in the cartilage of the knee often directly corresponded to damage to the ACL in animal models, but we could never really tell if the same thing was happening in people,” she says.
Beveridge and Fleming, her adviser, are hoping to do just that. T hey’ve taken 3-D images of dozens of patients’ knees, looking for minute differences in the joints after ACL reconstruction surgery in which the torn ligament is replaced with a graft of tendon. What they’ve found using XROMM, she says, has been surprising.
“It turns out even a millimeter difference in a joint’s mechanics can impact its health over time,” she says. “We think that some ACL reconstructions could leave knees at greater risk of increased stresses to the joint that can lead to osteoarthritis.”
“When people have the standard ACL surgery, their knee’s motion is no longer exactly where it’s supposed to be. We want to determine how close you need to be to keep the joint healthy over time,” Fleming adds. “Right now, nobody really knows that. If you have a pool of patients that have ACL reconstruction surgery, 60 percent move on to have arthritis. So even a small difference can be significant.”
If the researchers can understand why one patient’s joint stays healthy years after surgery while another’s is broken down and arthritic, it may help improve existing surgical techniques, and lower the number of patients who develop crippling arthritis in the joint years after reconstruction surgery. “To really know the best way to approach ACL treatment, we need to know basics of how the knee moves, how the joint is loaded, and what the biological response is,” Fleming says.
On some level, Brainerd says, gathering basic data about biomechanics was the primary goal she had in mind when developing XROMM. Whether she’s training it on human subjects or animals, her objective is the same: to gather as much data about basic biomechanics as possible, and make it possible for scientists worldwide to do so as well. With that in mind, the XROMM system’s design and software are entirely open source, freely available to any scientist who wants to use the technology. It’s a point of pride for Brainerd.
“Right now, there are a total of 28 systems out there that are based on our hardware design,” she says. “We want as many people as possible that have access to the hardware to be able to do XROMM.”
In the future, Brainerd wants to share far more than just software and specs with the research community. A s the number of XROMM-capable facilities increases worldwide, she says, she is promoting an online hub to collect and share the data they generate, so scientists can build off of each other’s progress.
“At this point my hope is scale. XROMM has gotten better and faster over the last 10 years, but we still only have data on a handful of species, and it’s been very time consuming to get it,” she says. A common database, however, would make it easier to bring in information from multiple studies and labs worldwide, creating a detailed picture of animal shape and diversity.
“I really just want a lot of people to use XROMM so we can do some broader comparisons—not just understanding how one animal works, but understanding how a whole bunch of animals work, how they evolved, and how they work differently,” she says. “Why do the ribs of different animals look so different? Why are skulls so different? To me, that’s what’s fundamentally interesting.”