Molecular details of sperm-egg fusion shed light on a fundamental mystery.
The fusion of a sperm cell with an egg cell is the very first step in the process that leads to new individuals in sexually reproducing species. Fundamental as this process may be, scientists are only now beginning to understand the complexities of how it works.
In a paper published in PLOS Biology, researchers have described the detailed structure of proteins that enable sperm-egg fusion in two different species: a flowering plant and a protozoan. The scientists hope that revealing the process in these species and their relatives might bring us a step closer to understanding it across sexual species, including humans and other vertebrates.
“It’s surprising to me that we still don’t know how a human sperm fuses with a human egg,” says Mark Johnson, PhD, an associate professor of biology and a study co-author. “One of the things we hope this paper will do is establish a structural signature for the proteins that make gamete fusion work in these species so that we might be able to look for it in species where those protein mechanisms are still unknown.”
Johnson has been working for years to understand gamete (sperm and egg) fusion. In the early 2000s, he identified a protein on the sperm membrane of the flowering plant species Arabidopsis thaliana that seemed to have some influence on the gamete fusion process in that species. His work showed that a mutation in the gene the produces the protein, known as HAP2, causes sperm become unable to fuse with the Arabidopsis egg.
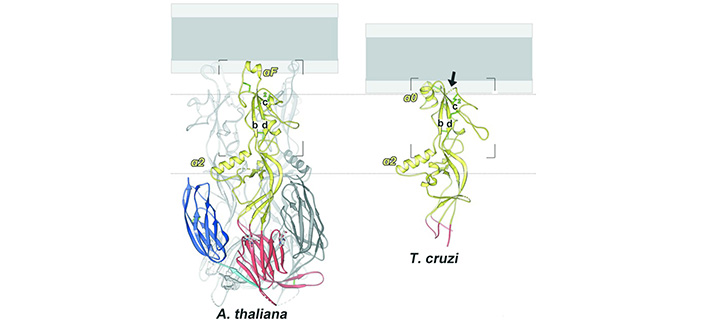
Johnson’s lab worked with the lab of Felix Rey, at Institut Pasteur in Paris, where graduate student Juliette Fedry resolved the structures of HAP2 proteins from two distantly related eukaryote species: Arabidopsis and Trypanosoma cruzi, a protozoan parasite. To resolve the structures, the team in Paris used x-ray crystallography, which involves crystallizing the proteins and then observing how the crystals scatter x-rays. The structure of the protein can be observed from the scattering pattern.
Jennifer Forcina PhD’18, a graduate student in Johnson’s lab, took advantage of the novel structural data to determine how HAP2 drives fertilization in flowering plants, defining the amino acids at the tip of the protein that insert into the egg membrane. The study found that while the basic structure of the HAP2 proteins from the two species was broadly similar, they had evolved to be different in key areas.
Read the full story here.