Mutation may cause early loss of sperm supply.
The loss of a single gene in male mice may offer clues in the complex puzzle of human male infertility.
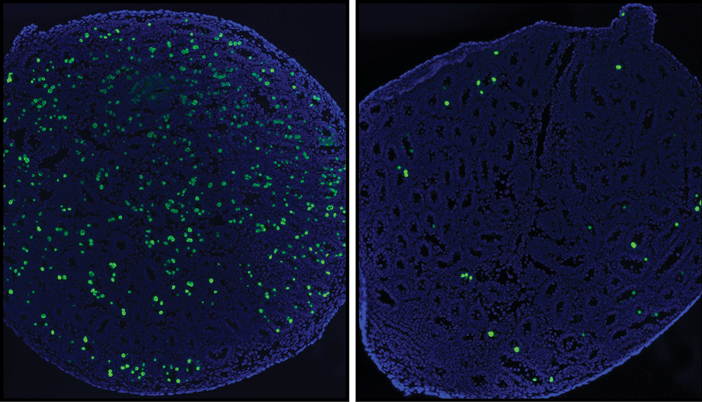
Male mice lacking the gene that makes the protein TAF4b have fewer progenitor cells at an embryonic stage of their reproductive development, Brown researchers reported in a recent study in the journal Stem Cells. Without these cells, the mice struggle to develop a robust stem cell infrastructure to sustain sperm production for the long term. Though they are fertile at first, the affected mice quickly deplete their limited sperm supply.
“What’s fascinating about these mice is they can reproduce,” says Richard Freiman, PhD, associate professor of medical science and senior author of the study. “Mice can usually reproduce until they are 2 years old, but these mice can only reproduce until they are 4 months old.”
TAF4b affects how genes are regulated and transcribed, and its absence has profound impacts on the reproductive system. In previous work, Freiman’s research group has shown that female mice without TAF4b are totally infertile and that their ovaries age prematurely. But in males, the effect proved more subtle.
Research led by postdoctoral researchers Lindsay Lovasco, PhD, and Eric Gustafson, PhD, found that in mice with TAF4b, progenitor cells for sperm in the male embryo arise and proliferate normally, laying the groundwork in the testes for a robust pool of spermatogonial stem cells to develop. Those stem cells go on to produce a renewable supply of sperm.
Mice without TAF4b have fewer progenitor cells and consequently fewer stem cells. They still produce sperm at first, but they can’t renew production for the long haul. Ultimately the testes, which develop normally, become unproductive and atrophy. What’s not yet clear is why the process fades out rather than just continuing, albeit at a very low level of productivity.
Not only do humans have a gene for TAF4b, but a study last year in the Journal of Medical Genetics suggests that it also matters for sperm count. That research reported that four Turkish brothers who carried a mutation in the TAF4b gene had low sperm counts. Their mutation was in the same region of their gene as the one Freiman’s team generated in the mice.
“The human implications are very exciting,” he says. “It is possible that those men, as teenagers, were able to make functional sperm.”
Certainly more research is needed, Freiman says, but if TAF4b mutation plays out in men the way it plays out in mice, his hope is that detecting the mutation in teenage boys could allow doctors to freeze their sperm so that if they want to have children when they are older, they could draw on that banked supply.
“This is why fundamental knowledge is so important,” Freiman says. “If we understand the process, we might be able to do something we couldn’t do before.”